Recalled Product
- Product Names: Oxylog 3000 Plus Emergency and Transport Ventilator
- Part Numbers: 5704811 and 5704813
- Product Material Numbers: See Recall Database Entry
- Distribution Dates: April 30, 2012 to June 13, 2022
- Devices Recalled in the U.S.: 300
- Date Initiated by Firm: June 12, 2023
Device Use
The Oxylog 3000 Plus Emergency and Transport Ventilator is used for people who require full or partial breathing assistance from a mechanical ventilator. Healthcare professionals use it during patient transport, either in an ambulance or aircraft or to move people using ventilators throughout the hospital and recovery room.
Reason for Recall
Draeger Medical is recalling the Oxylog 3000 Plus Emergency and Transport Ventilator after receiving reports that the device stopped ventilation because of a depleted battery, even after being re-connected to AC power. The ventilator may not automatically switch back to using AC power when it is plugged in and may continue using the battery until it is depleted, then stop providing ventilation. A battery alarm: “Charge int. battery” and “Int. battery discharged” does occur with this issue.
Stopped ventilation may cause difficulty breathing (respiratory distress), lack of oxygen (hypoxia), slow heartbeat (bradycardia), a sudden stop of the heart (cardiopulmonary arrest), other severe injuries, or death.
Draeger Medical reports six complaints, no injuries, and no deaths related to this issue.
Who May be Affected
- People who receive breathing support from the Oxylog 3000 Plus Emergency and Transport Ventilator
- Health care providers who provide breathing support care for patients with Oxylog 3000 plus Emergency and Transport Ventilator
What to Do
In June 2023, Draeger Medical sent affected customers an Urgent Medical Device Recall letter with the following recommendations:
- Make sure that the battery is always removed and reinserted or replaced after an occurrence of the “No int. battery charging” alarm message without removing the device from main power supply.
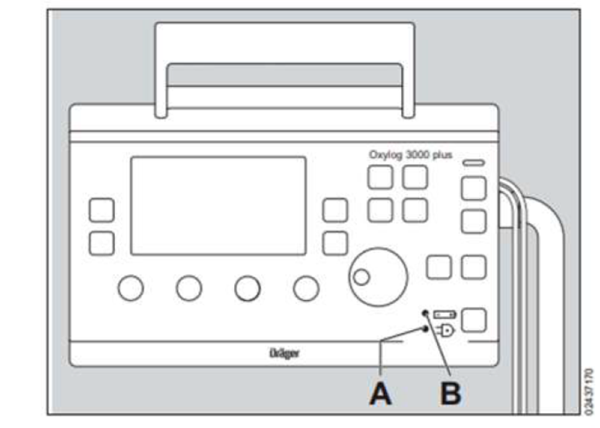
- Ensure the correct switchover by disconnecting and then reconnecting the device to an AC main power supply before using the device on battery power. Indicator A should display a green light, and indicator B should display a green or yellow light.
- If indicator B displays a red light, you should disconnect and reconnect or replace the battery.
- Devices can continue to be used safely as long as the precautions and actions above are taken.
- Ensure that all users and maintenance personnel of the products within the organization are made aware of the recall notice.
- Forward a copy of this information if products were provided to third parties.
- Complete and return the Customer Acknowledgment Card attached to the letter to confirm the information was received.
- Keep this information available until update measures have been completed.
- Contact Draeger Service Technical Support with questions about the operation of the Oxylog 3000 plus: Technical Support 1-800-437-2437, press 2 at the prompt, then 2, then 1, between the hours of 8 a.m. and 8 p.m. EST.
The letter also states that a local Draeger service representative will contact customers to arrange for a firmware update of the Printed Board Assembly Charger.